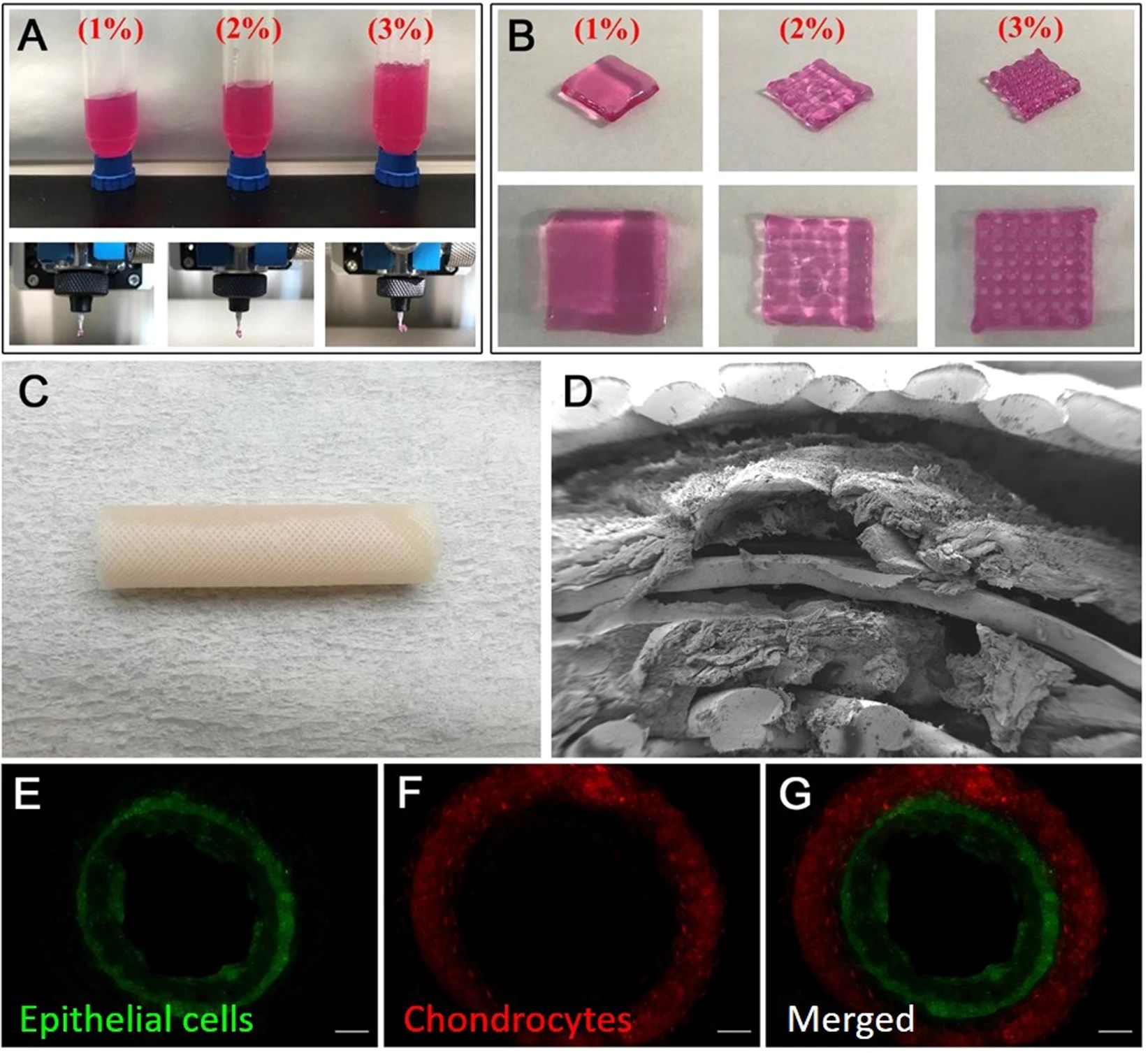
Summary
- Progress towards 3D-printed organs has been slow due to challenges like vascularization and cell viability.
- 3D bioprinting has successfully implanted hollow organs like tracheas and bladders, but solid organs present more technical challenges.
- Other promising alternatives to 3D bioprinting include xenotransplants, bionic organs, and growing organs in bioreactors.
About fourteen years ago, I sat down and watched a fascinating 2011 TED Talk by Professor Anthony Atala titled “Printing a human kidney”, showing off their progress towards creating organs with a 3D bioprinter.
That video blew my mind, and it seemed like we were just around the corner from solving our organ transplant crisis, but today we’re still dependent on donors to keep people on the recipient lists alive.
The Promise of Printed Organs Was Red-Hot
The idea that someone could create a replacement organ using a machine, and then implant it with no rejection risk or dangerous medications is obviously like a dream come true. I remember that the demos shown of these bioprinter prototypes needed disclaimers on them, so that desperate people on waiting lists for kidneys and other organs weren’t given false hope.
This was exciting, they said, but it would still be years before a 3D-printed kidney would go into a patient. Then things quietened down in the mainstream media. Behind the scenes, scientists and engineers were working tirelessly, and there’s no lack of funding in the world of regenerative medicine, but to the outside world it was easy to forget the whole thing.
It’s Harder Than It Sounds
The thing about technological development is that you can have moments where big breakthroughs happen, but they are interspersed with years of incremental problem-solving. Printing or otherwise creating something as complex as a kidney or a liver has a list of challenges a mile long. You can’t just make something that’s close to a kidney, you need to get it right.
While bioprinters “print” using living cells, a liver isn’t just a random mishmash of liver cells. The different cells and materials need to be arranged in exactly the right structure, so that the organ does its job.
Vascularization is a big problem, as solid organs have a complex structure of blood vessels that transport oxygen and nutrients through it. Printing layers of cells is easy enough, but printing vessels into those cells? Not so simple.
The cells in the printer also need to be kept alive throughout the process. A printed organ isn’t much use of the cells die during the printing process, so solutions to keep cells alive and healthy until the organ is complete and ready to either go on life support or be implanted is another major hurdle. It’s not at all like printing something in plastic or metal.
These printed organs also need to have at least the same structural integrity as natural ones, and there are things happening at the microscopic level that might not be replicable with current approaches to bioprinting.
These are just three major issues that I’m aware of, but I’m sure there’s a litany of issues that need to be solved before we get to human testing, and eventually mainstream adoption.
We’re Already 3D-Printing Body Parts and Hollow Organs
So you might have noticed that I’ve mainly talked about the printing of solid organs like the liver, kidneys, heart, and so on. That’s because when it comes to hollow organs or relatively simple structures in the body, we are already using medical-grade 3D printers to make replacement parts for patients.
The first 3D-printed organ implanted into a human happened all the way back in 1999! However, the scientists at Wake Forest University (the very same Anthony Atala) didn’t publish about it until 2006. The printed bladder was covered in the patients’ own cells, and as far as I can tell, it’s still doing its job.
Small victories like these keep piling up. In 2023, Korean scientists implanted the first ever 3D-printed trachea using bioprinted materials and stem cells from other people. While the trachea is only expected to last five or so years, the hope is that the patient’s body will regenerate the trachea using this implanted bioprinted part as scaffolding. Already, it seems, the patient’s body is generating new veins.
This is a big deal, especially in light of the major failure of synthetic tracheas, which resulted in the deaths of most recipients in the 2010s. 3D printing technology is also revolutionizing the replacement of bones. With printers that can print bones directly into the body, repair jawbones, and much more.
So, while you might not have heard big news about how we can print livers, kidneys, or other big-name organs, make no mistake about the impact that 3D bioprinting is already having on the lives of people.
One of the most promising approaches seems to be the idea of printing organ scaffolds using a biocompatible material, and then perfusing that scaffold with cultured organ cells made from a patient’s own stem cells. Over time, the biodegradable scaffold is absorbed and goes away, hopefully leaving a functioning organ behind.

Related
ISS Astronauts 3D Print Metal in Orbit for the First Time
Previous printers could only handle plastic feedstocks.
The Competing Alternatives
3D printing technology is by far not the only potential approach to making organs that can be implanted or transplanted into humans. There are other competing approaches which might end up being more viable, though it’s more likely that some of these methods will have to be combined to finally crack the problem once and for all.
One promising short-term solution to the donor organ shortage is to increase the number of organs that are viable. For example, with Ex Vivo Lung Perfusion (EVLP) lungs can be kept alive outside a human body, and then examined for disease or damage. In many cases, the lungs can be rehabilitated to the point where they are suitable for transplant, where before they would have to be destroyed. The same method is being evaluated for hearts, though the goal here is to minimize or prevent damage from the existing cold storage preservation methods. This ex vivo perfusion concept can be applied to most organs like livers and kidneys, but with varying levels of usefulness.
Another exciting possibility is that of xenotransplants. In other words, transplanting organs from non-human species into humans. This may sound like a bad idea, but by genetically modifying pigs, their organs can be made suitable for transplantation.At least that’s the theory, and this is obviously ongoing research. David Bennit, the first person to receive a modified pig heart transplant, died two months later, but progress continues. In 2024, a woman received a pig kidney.
She’s only the fifth person in the world to receive a pig organ genetically modified in this way, and everyone is obviously keen to see how it turns out. Towana Looney, the woman in question, differs from the other recipients in that she’s relatively healthy, kept that way by dialysis. All the other recipients have died within months, but so far things are looking good.
Then there’s the potential of making bionic organs. There have already been many artificial hearts over the years, though most of these have not kept their recipients alive for long, with the idea being that you could extend their lives until a donor heart could be found. However, the LVAD pump can supplement a failing heart, which either extends time until a transplant can happen, or allows the heart to rest and potentially recover.
Of course, carrying battery packs around and being one missed recharge away from serious trouble isn’t ideal! Perhaps one day these artificial systems could be powered by the body itself.
Related
How Will Future Implant Technology Get Power?
Implantable technology is becoming more common, but we need to find ways to power these gadgets in a sustainable way.
The last alternative to 3D bioprinting is growing organs outside the body using bioreactors. Basically, we’d want to get stem cells, then get them to become organ cells, which then have to multiply and grow into an organ just as they did to make the natural organs in our bodies. However, it needs pointing out that out organs start as tiny baby organs, and then grow to adult size over many years. So there’s a challenge in growing organs that can be used in adults!
How Long Will It Take?
It can seem that something big has been “around the corner” for years without anything substantive happening, or at least seemingly so. Experts in the field of regenerative medicine differ on timelines, but Professor Jennifer Lewis of Harvard University’s Wyss Institute for Biologically Inspired Engineering thinks that within a decade is about as soon as it gets.
Personally, in my very nonexpert opinion, I expect regenerated organs of some kind to pass human trials by the middle of this century. However, I would not be surprised if that turns out to be too pessimistic given that this research doesn’t happen in a vacuum, and breakthroughs in other areas can create periods of rapid progress.
It’s also worth considering that AI (Artifiical Intelligence) is pushing scientific research ahead by decades, helping us discover new proteins, new materials, and new ideas at a rapid pace. This might actually accelerate the timeline of organ research in ways we can’t predict right now. Our emerging machine intelligences might see things we’re missing or at least have the ability to simulate years of research quickly, to point scientists in the right way. Either way, I think the future of medical science is incredibly bright.